Description
CU-SP1 AUTO is an easy-to-use Fully Automated External Defibrillator (AED) that is small, light, and portable, and uses a battery. The AED automatically reads the patient’s electrocardiogram (ECG) and determines if a cardiac arrest that requires defibrillation has occurred, so that both medical professionals and the general public can easily operate it. Cardiac arrest can occur anytime to anyone at any place and may threaten the patient’s life if the appropriate CPR and/or electric shock with a defibrillator are not applied within a few minutes.
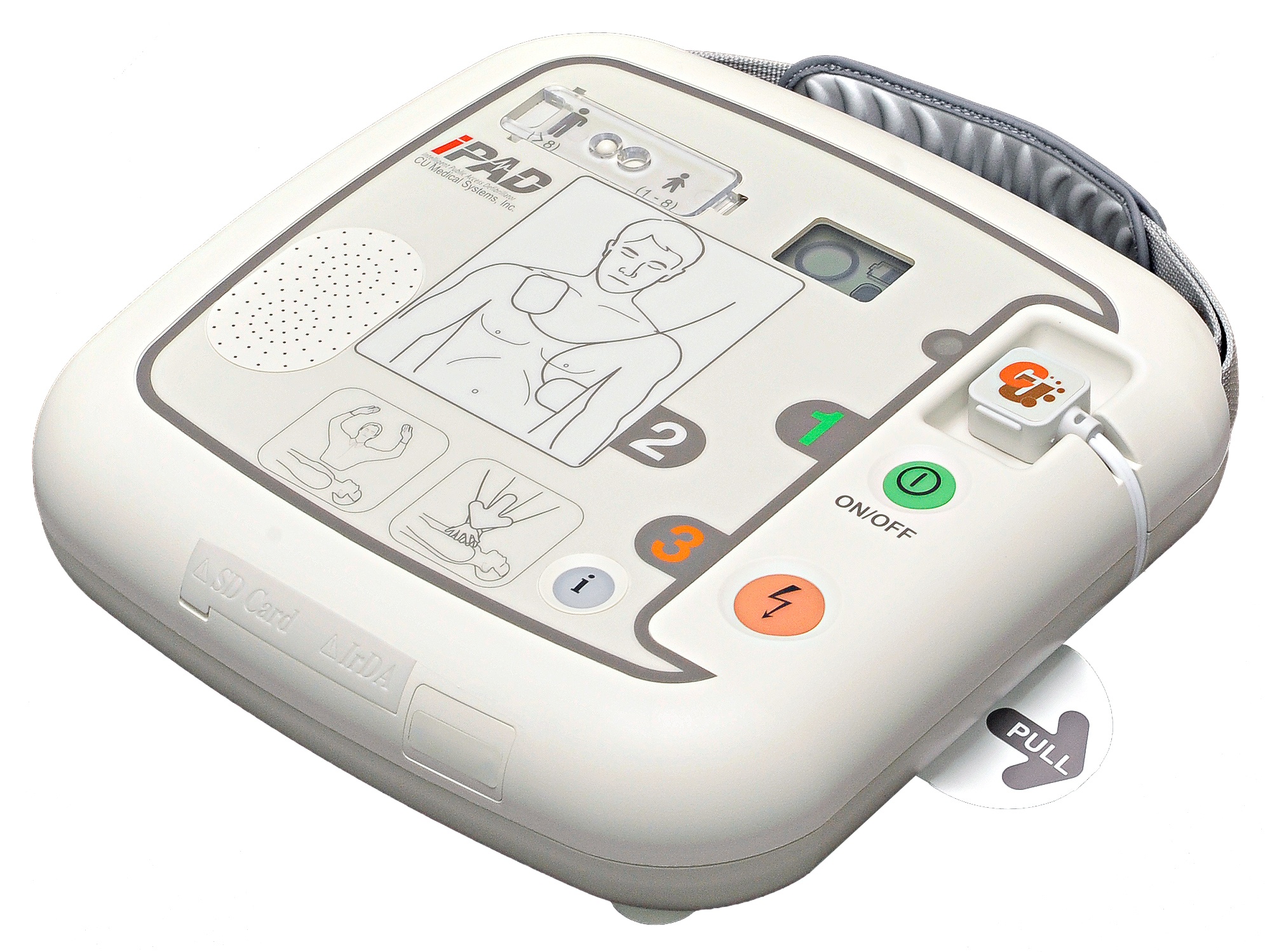
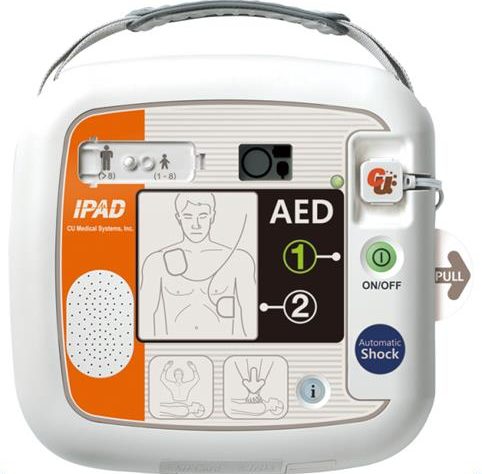
The IPAD CU-SP1 AUTO is a Fully automated external defibrillator (AED). If connected to a patient, the IPAD CU-SP1 AUTO automatically acquires and analyzes the electrocardiogram (ECG) of the patient for the presence of Ventricular Fibrillation or Ventricular Tachycardia (also known as shockable rhythms). If a shockable rhythm is detected, the device automatically charges itself. After charging is completed, the equipment delivers an electric shock automatically. The IPAD CU-SP1 AUTO is easy to use. It guides the you throughout a rescue operation using voice prompts and indicators (LED and graphical indicators). The IPAD CU-SP1 AUTO is small, light, highly portable, and battery powered. It is highly suitable for use in public, out-of-hospital settings.
Functions and Features
Ambient noise detection [Auto volume adjusting]
Easy-to-use & manage Electrode (Displaying Life expectancy of Electrode)
CPR guide & Detection.
Product Exterior
Dimension: 260 x 256 x 70 mm (Width x Length x Height)
Weight: Appr. 2.4kg (Including battery pack and pads) .
Defibrillator
Operating Mode: Semi-automated, Full-automated(NF1201)
Waveform: e-cube biphasic (Truncated exponential type)
Output Energy: 150 J at 50 A load for Adults, 50 J at 50 A load for Children .
Self-Diagnostic Test
Auto
Power On Self-Test, Run-time Self-Test
Daily, Weekly, and Monthly Self-Test
Manual
Battery Pack Insertion Test (done when the user inserts the battery pack into the battery pack compartment of the device).
ECG Analysis System
Fucntion: Determines the impedance of the patient and evaluates the ECG of the patient to determine whether it is shockable or non-shockable
Impedance Range: 25? ~ 175? .
Control Devices, Indicators, Voice Instructions
Control Devices Power Button, i-Button, Shock Button, Adult/Pediatric Selection Switch
Status LCD Displays device status, battery level and pads status
CPR Detection Indicator: Lights if CPR is detected; flashes if CPR is not detected..
Disposable Battery Pack
Battery Type: 12V DC, 4.2Ah LiMnO2, Disposable
Capacity: At least 200 shocks for a new battery or 8 hours of operating time at room temperature
Temperature
Operating: Temperature: 0? ~ 43? (32? ~ 109?)
Storage: Temperature: -20? ~ 60? (-4? ~ 140?).
Electrode
Adult
Electrode Area : 110cm2
Cable Length: Total 120cm
Pediatric
Electrode Area: 50cm2
Cable Length: Total 120cm.
Data Storage and Transfer
Internal Memory Data Capacity: 5 individual treatments, up to 3 hours per treatment
IrDA: For PC communications
SD Card External memory: Data may be copied from the internal memory to the SD Card
Standard Package
Device(1), Adult Pads(1), Disposable Battery(1), Carrying Case(1), User’s Manual(1).
Optional Accessories
Pediactric Pads(1), SD Card(1), CU-Expert software(1)